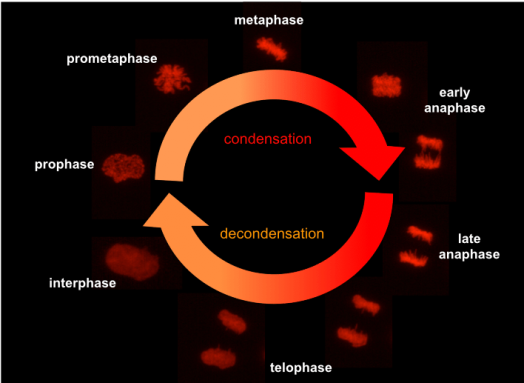
During cell division in eukaryotes, the cell nuclei suffer tremendous functional and structural reorganization. In animal and human cells, the nuclear envelope breaks down at the beginning of mitosis and the chromatin condenses to individualized chromosomes. Afterwards, the prototypical X-shaped chromosomes are captured, and their sister chromatids are segregated by the spindle apparatus. Then, the chromosomes decondense in an energy-dependent process, allowing the re-assembly of a functional nucleus.
Mitosis is undoubtedly a complex and fascinating process that has been studied for centuries, but still continues to amaze us. We are still profoundly ignorant about its true molecular magnitude and its influence on subsequent phases of the cell cycle and the life of the organism. Studying the molecular details of its spectacular dynamics is essential for the basic knowledge of the living matter, but also medically relevant since it is emerging as a therapeutic target in diverse diseases, including cancer and ageing.
molecular mechanisms of chromatin decondensation and nuclear reassembly in late stages of cell mitosis.
While the molecular mechanisms involved in chromatin compaction and segregation during early mitosis received attention and are relatively well investigated, much less is known about the mitotic chromatin decondensation and the subsequent nuclear reformation. Recent evidence suggests that mitotic chromatin decondensation and nuclear reformation are multistep processes deeply interdependent. However, we have still little knowledge about the molecular mechanisms that rule them and their influence in functional aspects of the nucleus during interphase. Furthermore, we are largely unaware of potential medical implications in human pathologies. Therefore, our research is focused on understanding from the molecular point of view on how and why chromosomes decondense and their involvement in the nuclear reformation after mitosis.
mitotic progression in hematopoietic malignancies
Myeloid malignancies are a heterogeneous set of illnesses that affect the homeostasis of hematopoietic stem and progenitor cells (HSPCs). Defective cell differentiation, faulty self-renewal, and cell hyperproliferation are hallmarks of these genetic and epigenetic disorders. Among those malignancies, myeloproliferative neoplasms can evolve to secondary acute myeloid leukemia. However, the molecular mechanism of this phase transition towards a worse prognosis is still poorly understood. It is likely that many mutations typical in these malignancies impact different stages of mitotic progression and regulation to trigger the transition from chronic to acute phases of the diseases. However, the mitotic progression of myeloblastic cells remains largely unexplored. Therefore, our focus here is to understand how and which genetic changes in hematopoietic malignancies perturb the molecular mechanisms of mitotic progression to gain knowledge of direct clinical relevance. This will help to define cell-cycle-specific therapeutic windows, selective pharmacological targets and gen-to-drug synthetic approaches.
our approach
We face these research challenges by combining different methodologies in biochemistry and molecular cell biology with advanced optical microscopy and image analysis. For this, we count on the very well-equipped infrastructure of our institute and the cutting edge technology provided for the manifold facilities on the campus (see affiliations and locations).

As microscopists, we are part of the Advanced Light Microscopy Aachen network and our workhorse (above) is a Nikon Ti2-E with Crest X-Light V2 Spinning Disk microscope. A very flexible inverted fluorescence platform geared for medium/high-throughput time-lapse phenotypic screenings and high-resolution imaging of living cells.
affiliations and locations
Our group is located at the Institute of Biochemistry and Molecular Cell Biology at the Medical School, RWTH Aachen University in Aachen (Germany)
