science outreach
The cell nucleus is the organelle that contains the vast majority of the genes that support life in eukaryote organisms. Cells in eukaryotes present the greatest structural complexity. Human cells contain a nucleus with extraordinarily complex architecture, whose structure helps to maintain multiple functions. One of these functions is maintaining the integrity of the genetic material (DNA) to avoid mutations with potential consequences for the health of the organism, such as degenerative diseases or cancer. The DNA molecules, which contain the sequence of all our genes, bind a multitude of different proteins that protect, control, and organize them. This microscopic tangle of DNA and proteins is called chromatin and a porous membrane, also involved in the protection and control of the genome, surrounds it. In human cells, chromatin is composed of forty-six independent blocks called chromosomes.
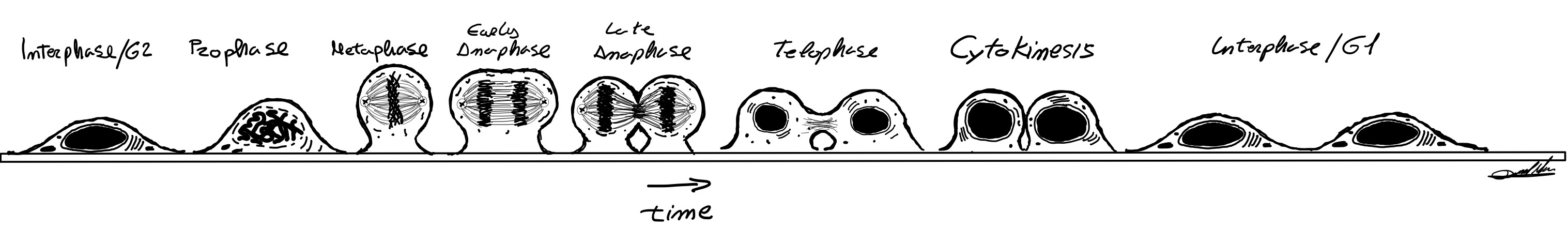
The life of a cell is conceptually symbolized by “the cell cycle”. Two daughter cells are born from the division, called mitosis, of a previous cell. Then these daughter cells pass to a phase (called interphase) where they grow in size, replicate their genetic material, and perform their organic functions until they enter again in mitosis. The mitotic phase has been classically subdivided in several sub-phases attending to morphological, cytological and molecular characteristics of the whole cell and the chromatin organization.
During mitosis, the interior of the cell undergoes tremendous reorganization, especially the complex structure of the nucleus. The goal is to ensure that each daughter cell receives exactly one copy of the genetic material. To do this, the nuclear envelope is broken down and the chromatin forms typical individualized X-shaped chromosomes. These chromosomes are then captured by the spindle apparatus and their sister parts (named chromatids) separated. The spindle apparatus is a complex, dynamic molecular machinery that exerts pulling forces to mechanically separate the chromosomes. Once separated, the chromosomes decondense while merging into a large mass of chromatin and the nuclear envelope wraps the chromatin up, reassembling a functional nucleus. Finally, the two new cell bodies are separated.
The cells of an organism organize themselves into tissues. The process of development, growth, and life of an organism will largely depend on whether some of these cells divide to generate others. Stem cells are those that always divide, but in a controlled way. Most of the cells produced by the stem cells normally do not divide any more or, if they do, it is very little and in very specific cases. This allows keeping stable structures and functions of the different tissues and organs. When a group of cells escapes from the tight control of its genetic program and proliferate abnormally, it can lead to various pathologies, including myeloid diseases, cancers, degenerative diseases and accelerated ageing. Mutations in certain key genes are in the base of the molecular causes that lead to developing these diseases. However, we still do not know much about how, when and which molecular mechanisms fail, ultimately causing disease.
We know that in many cancers the process of mitosis has serious flaws, which are a side effect of the mutations that cause uncontrolled cell proliferation, aggravating the pathology. However, the importance of mitotic progression in hematopoietic malignancies, degenerative disease, and ageing remains largely unexplored. We do not know, in most of the cases, whether and how mutations that cause these diseases affect mitotic progression. As molecular cell biologists and microscopists, we routinely use image analysis of microscopic images of living cells to identify the nature of mitotic defects and elucidate basic molecular mechanisms of cell division. One of the main objectives of our work, as part of a medical faculty, is to unravel whether mitotic defects are present in human pathologies and generate a comprehensive knowledge of the pathological disease mechanisms. Our findings will join the collective effort to facilitate future therapeutic intervention.